Chemistry, 09.03.2021 03:20 1deanxcas1
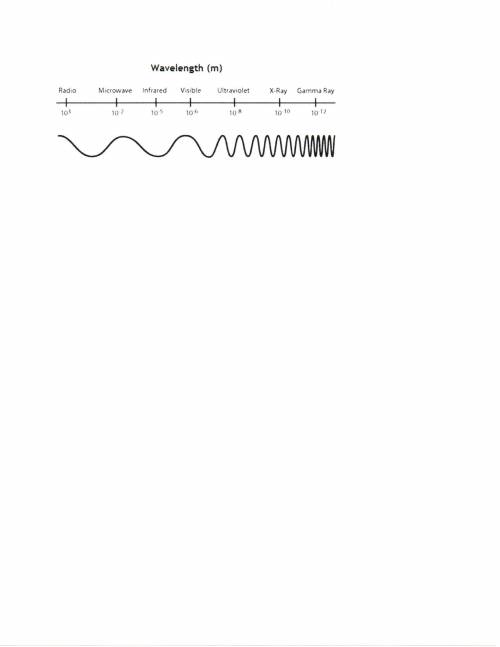
14) A wavelength of radiation has a frequency of 2.10 x 1014 Hz. What is the wavelength of this radiation in nm, and determine the type of radiation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3


Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
14) A wavelength of radiation has a frequency of 2.10 x 1014 Hz. What is the wavelength of this radi...
Questions



History, 01.09.2021 03:20

Biology, 01.09.2021 03:20


Mathematics, 01.09.2021 03:20

Biology, 01.09.2021 03:20


Physics, 01.09.2021 03:20



Mathematics, 01.09.2021 03:20



History, 01.09.2021 03:20

Mathematics, 01.09.2021 03:20

Mathematics, 01.09.2021 03:20


History, 01.09.2021 03:20

Medicine, 01.09.2021 03:20