
Chemistry, 09.03.2021 03:10 shaylaahayden45061
PLEASE HELP I'VE BEEN STUCK ON THIS FOR 2 HOURS
Calculate the mass of water produced from the reaction between 8.50 g of Hydrogen gas and 85.0 grams of Oxygen gas.
(You're going to need a periodic table, and this is Mass to Mass conversion)
Equation: 2H2O->2H2 + O2
I got the answer 180.7 gH202 but it's not correct.
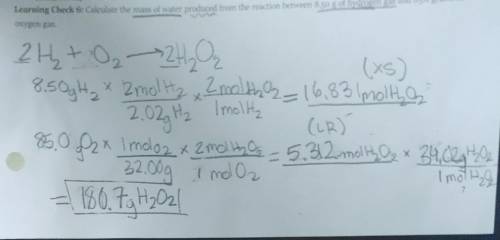

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 23:00
What extra step distinguishes fermentation from glycolysis
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

You know the right answer?
PLEASE HELP I'VE BEEN STUCK ON THIS FOR 2 HOURS
Calculate the mass of water produced from the react...
Questions

English, 01.07.2019 15:00

Mathematics, 01.07.2019 15:00

Social Studies, 01.07.2019 15:00


Mathematics, 01.07.2019 15:00

English, 01.07.2019 15:00





History, 01.07.2019 15:00

Advanced Placement (AP), 01.07.2019 15:00


History, 01.07.2019 15:00

Mathematics, 01.07.2019 15:00

History, 01.07.2019 15:00

English, 01.07.2019 15:00

Spanish, 01.07.2019 15:00

Mathematics, 01.07.2019 15:00

History, 01.07.2019 15:00




