
Chemistry, 09.03.2021 03:10 robert7248
The data table shows the rate of the decomposition reaction of hydrogen
peroxide, H202. Which statement explains how the rate of the reaction
changes over time?
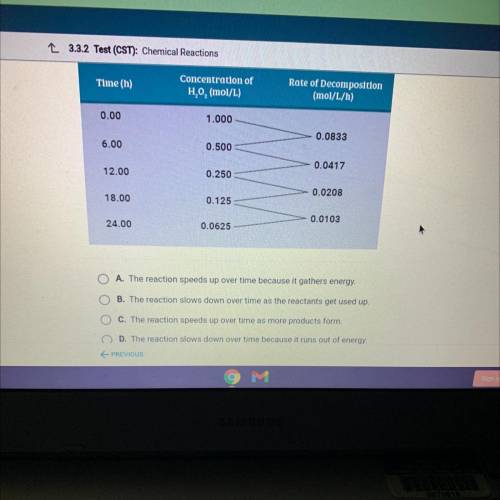

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1


Chemistry, 23.06.2019 07:30
Achemist at a pharmaceutical company is measuring equilibrium constants for reactions in which drug candidate molecules bind to a protein involved in cancer. the drug molecules bind the protein in a 1: 1 ratio to form a drug-protein complex. the protein concentration in aqueous solution at 25 ˚c is 1.74 x10-6 m . drug a is introduced into the protein solution at an initial concentration of 2.00 x10-6m. drug b is introduced into a separate, identical protein solution at an initial concentration of 2.00 x10-6m. at equilibrium, the drug a-protein solution has an a-protein complex concentration of 1.00 x10-6m, and the drug b solution has a b-protein complex concentration of 1.40 x10-6m.a. calculate the kc value for the a-protein binding reaction.b. calculate the kc value for the b-protein binding reaction.c. assuming that the drug that binds more strongly will be more effective, which drug is the better choice for further research?
Answers: 1

Chemistry, 23.06.2019 10:30
The element chlorine has two stable isotopes, chlorine-35 with a mass of 34.97 amu and chlorine-37 with a mass of 36.95 amu. from the atomic weight of cl = 35.45 one can conclude that:
Answers: 2
You know the right answer?
The data table shows the rate of the decomposition reaction of hydrogen
peroxide, H202. Which state...
Questions













Mathematics, 28.05.2021 01:40





Mathematics, 28.05.2021 01:40




