

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3
You know the right answer?
A chemist reacts 30.0 mL of 5.6 M HCl with an excess of Mg(OH)2 How many grams of magnesium will be...
Questions

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

English, 09.09.2020 23:01

Biology, 09.09.2020 23:01

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Physics, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

Biology, 09.09.2020 23:01

History, 09.09.2020 23:01

English, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

History, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

English, 09.09.2020 23:01

History, 09.09.2020 23:01

Mathematics, 09.09.2020 23:01

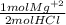 = 0.084 mol Mg⁺²
= 0.084 mol Mg⁺²

