
Chemistry, 08.03.2021 15:00 sihamabdalla591
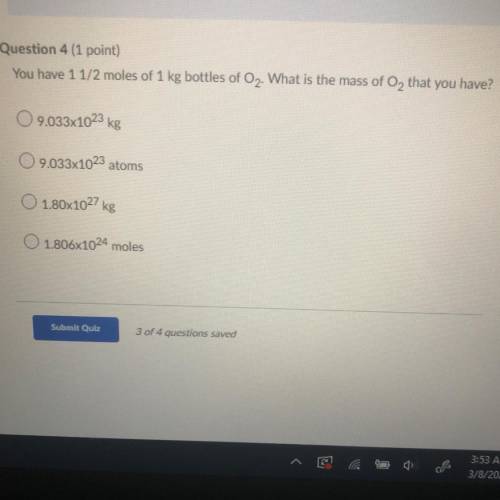
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg
B. 9.033x10^23 atoms
C. 1.80x10^27 kg
D. 1.806x10^24 moles

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:50
In a popular classroom demonstration, solid sodium is added to liquid water and reacts to produce hydrogen gas and aqueous sodium hydroxide. part a write a balanced chemical equation for this reaction. express your answer as a chemical equation. identify all of the phases in your answer.
Answers: 3

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1


Chemistry, 22.06.2019 07:20
Part b: study of equilibrium on solubility: mg(oh)2(s) ⇌ mg2+(aq) + 2 oh–(aq) cloudy clear (pink) 7. a. b. 8. a. b. 9. 10. 11. 12. when adding concentrated hydrochloric acid, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 7a. you should indicate which ion was added to or removed from the equilibrium mixture. when adding edta, how did the appearance of the equilibrium mixture change? the change in appearance indicated a shift in the point of equilibrium. in which direction did the equilibrium shift? (l) left (r) right explain your answer to question 8a. you should indicate which ion was added to or removed from the equilibrium mixture. upon heating in which direction is the equilibrium shifting? upon cooling in which direction is the equilibrium shifting? is the forward reaction a. endothermic explain your answers to questions 9, 10, and 11. (l) left (r) right (l) left (r) right b. exothermic
Answers: 1
You know the right answer?
You have 1 1/2 moles of 1 kg bottles of O2. What is the mass of O2 that you have?
A. 9.033x10^23 kg...
Questions

Chemistry, 28.02.2021 06:00

Mathematics, 28.02.2021 06:10





Mathematics, 28.02.2021 06:10



Mathematics, 28.02.2021 06:10


English, 28.02.2021 06:10

Mathematics, 28.02.2021 06:10

Mathematics, 28.02.2021 06:10


Spanish, 28.02.2021 06:10

Spanish, 28.02.2021 06:10

Mathematics, 28.02.2021 06:10

Mathematics, 28.02.2021 06:10

Mathematics, 28.02.2021 06:10



