
Chemistry, 08.03.2021 14:00 roseemariehunter12
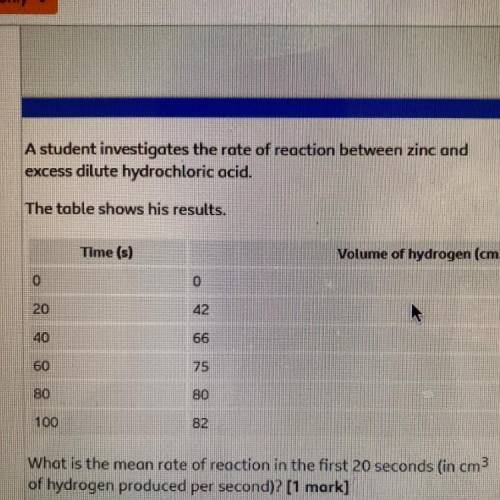
A student investigates the rate of reaction between zinc and
excess dilute hydrochloric acid.
The table shows his results.
Time (s)
Volume of hydrogen (cm3)
0
0
20
42
40
66
60
80
80
100
82

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 23.06.2019 05:00
Which of the following describes qualitative data? a) recording the temperature of a solid as it is warmed. b) noting the color of a solution as it is heated. c) measuring the volume of an object by water displacement. d) taking the mass of an object using a balance.
Answers: 2
You know the right answer?
A student investigates the rate of reaction between zinc and
excess dilute hydrochloric acid.
...
...
Questions

Chemistry, 02.10.2021 14:00

Health, 02.10.2021 14:00


Biology, 02.10.2021 14:00

Social Studies, 02.10.2021 14:00

Social Studies, 02.10.2021 14:00


Chemistry, 02.10.2021 14:00

Social Studies, 02.10.2021 14:00

Geography, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00

Mathematics, 02.10.2021 14:00

Social Studies, 02.10.2021 14:00



Biology, 02.10.2021 14:00







