
Chemistry, 08.03.2021 01:30 4tazaouiamine1r
< Question 16 of 21 >
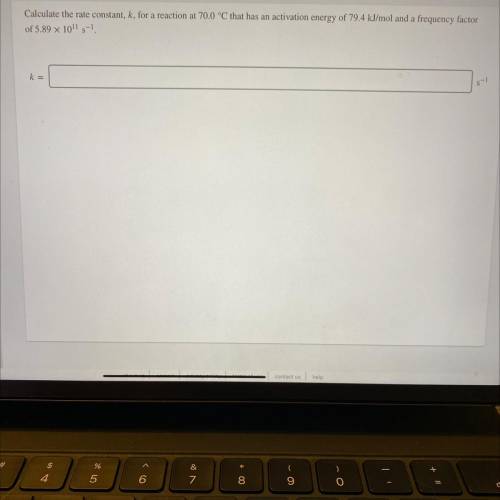
Calculate the rate constant, k, for a reaction at 70.0 °C that has an activation energy of 79.4 kJ/mol and a frequency factor
of 5.89 x 1011 5-1
s-1
k=

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Which orbitals form a pi bond? a.the s orbital and three p orbitals b.the s orbital and two p orbitals c.overlapping p orbitals d.overlapping hybrid orbitals
Answers: 2

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1
You know the right answer?
< Question 16 of 21 >
Calculate the rate constant, k, for a reaction at 70.0 °C that has an a...
Questions

History, 20.09.2019 11:50





Mathematics, 20.09.2019 11:50

Arts, 20.09.2019 11:50



Social Studies, 20.09.2019 11:50


Mathematics, 20.09.2019 11:50

Biology, 20.09.2019 11:50


History, 20.09.2019 11:50

Social Studies, 20.09.2019 11:50


Mathematics, 20.09.2019 11:50





