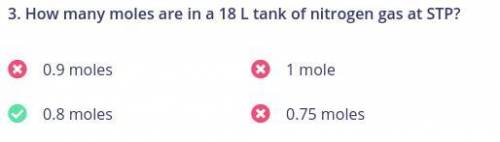
I have a quiz yall help
How many moles are in a 18 L tank of nitrogen gas at STP?...

Chemistry, 07.03.2021 14:00 mimithurmond03
I have a quiz yall help
How many moles are in a 18 L tank of nitrogen gas at STP?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 03:10
The covalent compound acetylene, which is the fuel of the oxyacetylene torch used by welders, has the molecular formula c2h2. the covalent compound benzene, a commercial solvent, has the molecular formula c6h6 each of these covalent compounds contains carbon and hydrogen atoms in a one-to-one ratio. would it be correct to write the chemical formulas of each as ch? explain.
Answers: 1

Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2
You know the right answer?
Questions


Mathematics, 10.11.2020 01:30




Mathematics, 10.11.2020 01:30


Mathematics, 10.11.2020 01:30


Mathematics, 10.11.2020 01:30

History, 10.11.2020 01:30






Mathematics, 10.11.2020 01:30

Mathematics, 10.11.2020 01:30


Mathematics, 10.11.2020 01:30