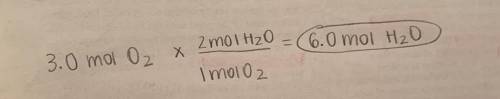Use the balanced equation below
O2 + 2H2 > 2H2O
1. How many moles of hydrogen are ne...

Chemistry, 07.03.2021 01:00 markarianlaura1
Use the balanced equation below
O2 + 2H2 > 2H2O
1. How many moles of hydrogen are needed to complete the reaction?
2. If 3.0 moles of oxygen are present with excess hydrogen, how many moles of water are formed?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
Questions

English, 03.03.2020 06:05