
Chemistry, 06.03.2021 06:20 12233445566
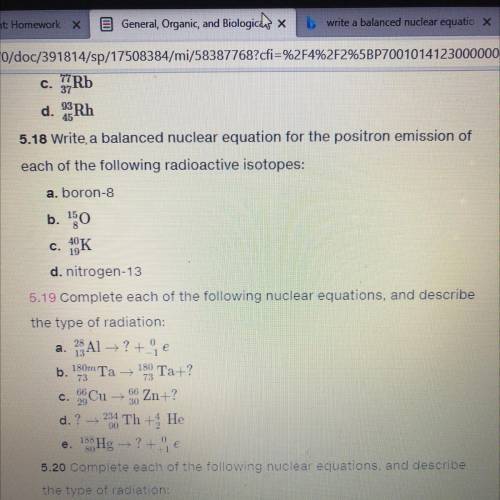
Write a balanced nuclear equation for the positron emission of each of the following radioactive isotopes:
a. boron-8
b. 15/8 10
c. 40/19 K
d. nitrogen-13

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3



Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Write a balanced nuclear equation for the positron emission of each of the following radioactive iso...
Questions


Physics, 23.09.2021 01:50



Mathematics, 23.09.2021 01:50

Health, 23.09.2021 01:50

Mathematics, 23.09.2021 01:50

Mathematics, 23.09.2021 01:50


Mathematics, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00


Mathematics, 23.09.2021 02:00


Mathematics, 23.09.2021 02:00

Chemistry, 23.09.2021 02:00

Mathematics, 23.09.2021 02:00





