
Chemistry, 06.03.2021 01:00 hi510hello
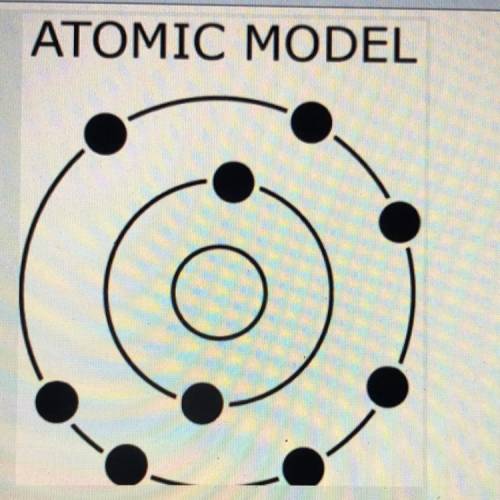
The diagram to the right shows an atomic model.
Based upon the arrangement of the electrons, which property would this atom most likely
have compared to iodine (I)?
(A)large atomic radius
(B)small nuclear charge
(C)low first ionization energy
(D)high electronegativity value

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 02:00
Which of the following is not a good technique for managing used oil? a) have specific, labeled catch pans available for technicians who are collecting oil b) spills in your shop and any releases on pavement or outside should be poured down a drain c) do not use oil containers for antifreeze or other non-similar fluids d) be prepared for oil spills with the proper absorbents
Answers: 1

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2
You know the right answer?
The diagram to the right shows an atomic model.
Based upon the arrangement of the electrons, which...
Questions















Mathematics, 10.03.2020 16:56

Social Studies, 10.03.2020 16:56

Mathematics, 10.03.2020 16:56

Mathematics, 10.03.2020 16:56




