
Chemistry, 06.03.2021 01:00 alecnewman2002
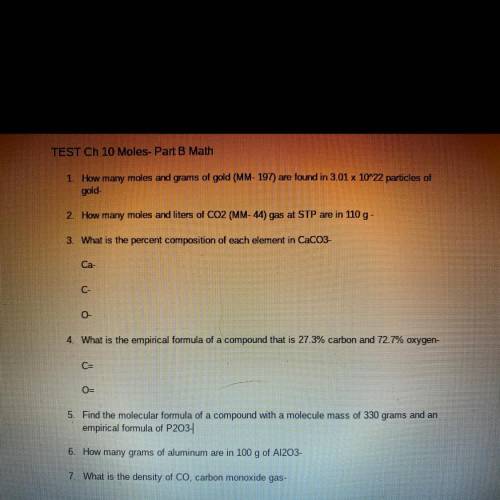
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^22 particles of
gold-
2. How many moles and liters of CO2 (MM-44) gas at STP are in 110 g -
3. What is the percent composition of each element in CaCO3-
Ca-
C-
0
4. What is the empirical formula of a compound that is 27.3% carbon and 72.7% oxygen-
C=
O-
5. Find the molecular formula of a compound with a molecule mass of 330 grams and an
empirical formula of P203-
6. How many grams of aluminum are in 100 g of A1203-
7. What is the density of Co, carbon monoxide gas-

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The pressure in a fluid is affected by which characteristics of that fluid
Answers: 1

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2
You know the right answer?
TEST Ch 10 Moles- Part B Math
1. How many moles and grams of gold (MM- 197) are found in 3.01 x 10^...
Questions

Mathematics, 04.01.2022 16:00

Mathematics, 04.01.2022 16:00


Mathematics, 04.01.2022 16:10

SAT, 04.01.2022 16:10



Health, 04.01.2022 16:10

SAT, 04.01.2022 16:10

Mathematics, 04.01.2022 16:10


SAT, 04.01.2022 16:10


SAT, 04.01.2022 16:10




SAT, 04.01.2022 16:10





