Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
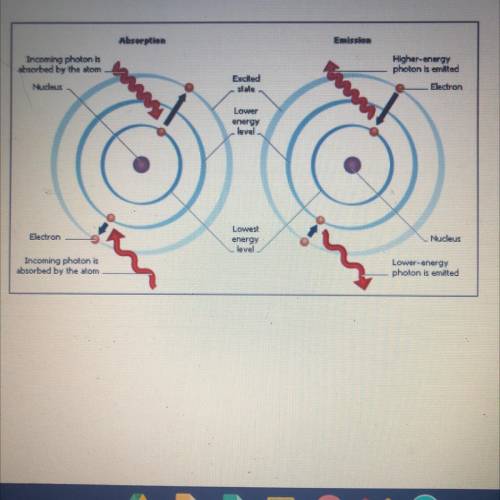
Q.1 When a metal is heated, what
happens to the electrons in
the atoms?
Q.2 What is th...
the atoms?
Q.2 What is th...
Questions

Mathematics, 26.10.2020 04:40


Mathematics, 26.10.2020 04:40

Mathematics, 26.10.2020 04:40

English, 26.10.2020 04:40


Mathematics, 26.10.2020 04:40

Mathematics, 26.10.2020 04:40

Mathematics, 26.10.2020 04:40

Mathematics, 26.10.2020 04:40


Mathematics, 26.10.2020 04:40




Business, 26.10.2020 04:40