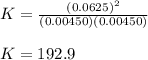Chemistry, 05.03.2021 14:00 hoopstarw4438
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. Determine the balanced equation and write the equilibrium expression
b. Determine the K eq
c. Will this process favor the reactants or products at equilibrium?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

You know the right answer?
At equilibrium at 2500K, [HCl]=0.0625M and [H2]=[Cl2]=0.00450M for the reaction H2+Cl2 ⇌ HCl.
a. De...
Questions



History, 12.04.2021 20:50

English, 12.04.2021 20:50




Biology, 12.04.2021 20:50





Physics, 12.04.2021 20:50

Mathematics, 12.04.2021 20:50

English, 12.04.2021 20:50


Mathematics, 12.04.2021 20:50

English, 12.04.2021 20:50


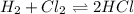
![K=\frac{[HCl]^2}{[H_2][Cl_2]}](/tpl/images/1171/4277/48ea9.png)