CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
...

Chemistry, 05.03.2021 07:00 HaydenSturgis1
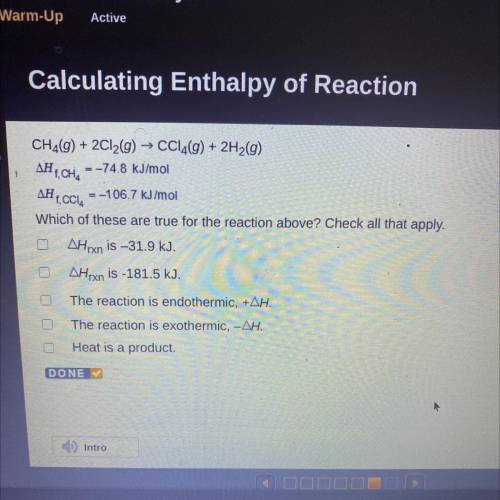
CH2(g) + 2Cl2(g) → CCl4(g) + 2H2(g)
AH T, CH, = -74.8 kJ/mol
AH 1. CCI, = -106.7 kJ/mol
Which of these are true for the reaction above? Check all that apply.
AHrxn is –31.9 kJ.
AHrxn is -181.5 kJ.
The reaction is endothermic, +AH.
The reaction is exothermic, -AH.
Heat is a product.
PLEASE HELP IM BEGGING AND I WILL GIVE BRAINLIEST

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 20:00
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1
You know the right answer?
Questions







Advanced Placement (AP), 26.08.2020 21:01




Health, 26.08.2020 21:01





Biology, 26.08.2020 21:01

Mathematics, 26.08.2020 21:01


Spanish, 26.08.2020 21:01

Mathematics, 26.08.2020 21:01



