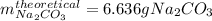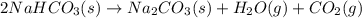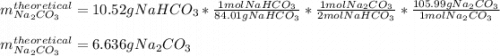Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 23.06.2019 05:40
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2


You know the right answer?
A student heats 10.52 g of sodium hydrogen carbonate in a crucible until the compound completely dec...
Questions


Mathematics, 10.07.2019 08:30

Mathematics, 10.07.2019 08:30

History, 10.07.2019 08:30

Social Studies, 10.07.2019 08:30

History, 10.07.2019 08:30

History, 10.07.2019 08:30

Social Studies, 10.07.2019 08:30




Biology, 10.07.2019 08:30



Mathematics, 10.07.2019 08:30