
Chemistry, 04.03.2021 23:40 kingjames82
Question 7 (1 point)
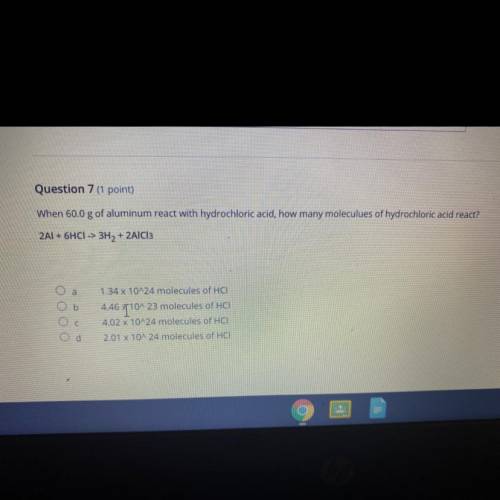
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of hydrochloric acid react?
2Al + 6HCI -> 3H2 + 2AlCl3
a
b
ОООО
1.34 x 10^24 molecules of HCI
4.46 T10^23 molecules of HCI
4.02 x 10^24 molecules of HCI
2.01 X 10^24 molecules of HCI

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:10
How do you identify the anode on a power source such as a battery? how do you identify the cathode? how are terms anion and cation?
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
Question 7 (1 point)
When 60.0 g of aluminum react with hydrochloric acid, how many moleculues of h...
Questions

Chemistry, 13.11.2020 20:00




Chemistry, 13.11.2020 20:00



History, 13.11.2020 20:00


Chemistry, 13.11.2020 20:00

Biology, 13.11.2020 20:00



Chemistry, 13.11.2020 20:00

Mathematics, 13.11.2020 20:00


Health, 13.11.2020 20:00

Arts, 13.11.2020 20:00


English, 13.11.2020 20:10



