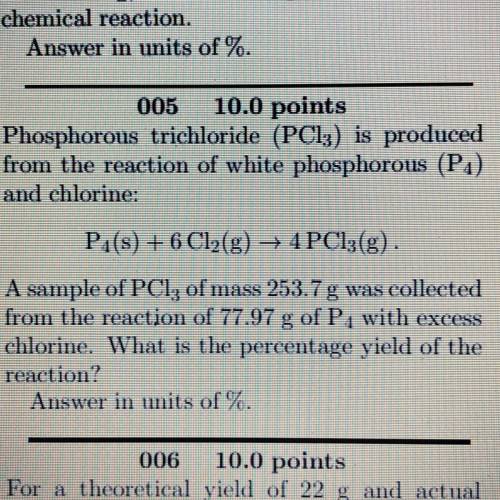
Phosphorous trichloride (PC13) is produced
from the reaction of white phosphorous (P1)
and ch...

Chemistry, 04.03.2021 22:10 Jamilia561
Phosphorous trichloride (PC13) is produced
from the reaction of white phosphorous (P1)
and chlorine:
Pa(s) + 6 Cl2(g) → 4PC13(g)
A sample of PCl, of mass 253.7 g was collected
from the reaction of 77.97 of P, with excess
chlorine. What is the percentage yield of the
reaction?
Answer in units of %

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:20
Aluminum reacts with chlorine gas to form aluminum chloride via the following reaction: 2al(s)+3cl2(g)→2alcl3(s) what is the maximum mass of aluminum chloride that can be formed when reacting 32.0 g of aluminum with 37.0 g of chlorine? express your answer to three significant figures and include the appropriate units.
Answers: 2

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3
You know the right answer?
Questions

Physics, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01



Mathematics, 09.09.2020 22:01



English, 09.09.2020 22:01







Social Studies, 09.09.2020 22:01

Social Studies, 09.09.2020 22:01

Social Studies, 09.09.2020 22:01

Mathematics, 09.09.2020 22:01




