
Chemistry, 04.03.2021 21:00 brooket30057
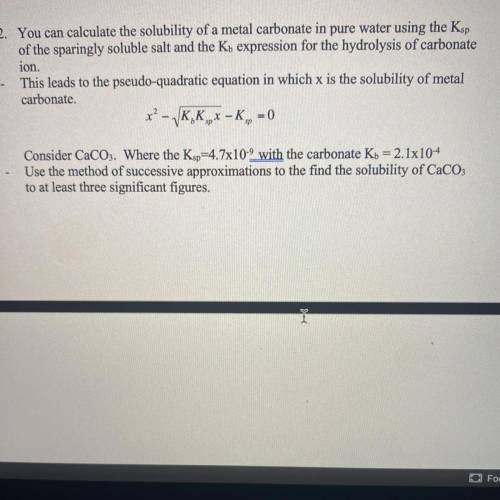
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparingly soluble salt and the Kb expression for the hydrolysis of carbonate
ion.
This leads to the pseudo-quadratic equation in which x is the solubility of metal
carbonate.
*? - VK, K,- K,, -
= 0
Consider CaCO3. Where the Ksp=4.7x10-9 with the carbonate Kb = 2.1x10-4
Use the method of successive approximations to the find the solubility of CaCO3
to at least three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 07:00
If there is any 12 to 14 girls that need a boyfriend just follow me and let me know
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
2. You can calculate the solubility of a metal carbonate in pure water using the Ksp
of the sparing...
Questions

Mathematics, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40


Mathematics, 19.11.2020 01:40




Social Studies, 19.11.2020 01:40

History, 19.11.2020 01:40

English, 19.11.2020 01:40


English, 19.11.2020 01:40



Geography, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40



History, 19.11.2020 01:40

Mathematics, 19.11.2020 01:40



