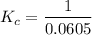Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2Hg (l) + O2 (g) → 2HgO (s)
At a certain temperature, a chemist finds that a reaction vessel containing a mixture of mercury(II) oxide, mercury, and oxygen at equilibrium has the following composition:
compound amount
Hg 14.7g
O2 13.4g
HgO 17.8g
Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Asample of gas occupies 17 ml at –112°c. what volume does the sample occupy at 70°c a. 10.6 ml b. 27 ml c. 36 ml d. 8.0 ml you
Answers: 1


Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1
You know the right answer?
Mercury(II) oxide decomposes to form mercury and oxygen, like this:
2Hg (l) + O2 (g) → 2HgO (s)
Questions

Mathematics, 19.01.2021 04:40

Mathematics, 19.01.2021 04:40

Social Studies, 19.01.2021 04:40



Mathematics, 19.01.2021 04:40





Social Studies, 19.01.2021 04:40



Mathematics, 19.01.2021 04:40

Mathematics, 19.01.2021 04:40



Mathematics, 19.01.2021 04:40


Mathematics, 19.01.2021 04:40

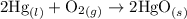
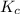 contains aqueous an dgas species only.
contains aqueous an dgas species only. ![$K_c=\frac{1}{[O_2]}$](/tpl/images/1168/7990/eab15.png) ............(1)
............(1)![$[O_2]= \frac{n_{O_2}}{V_{soln}}$](/tpl/images/1168/7990/80b5d.png) ................... (2)
................... (2)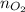 = no. of moles of oxygen gas (mol)
= no. of moles of oxygen gas (mol)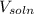 = volume of solution (L)
= volume of solution (L)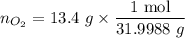
![$[O_2] =\frac{0.418 \ \text{mol}}{6.9 \ \text{L}}$](/tpl/images/1168/7990/d9674.png)