
Chemistry, 04.03.2021 19:50 SoccerHalo
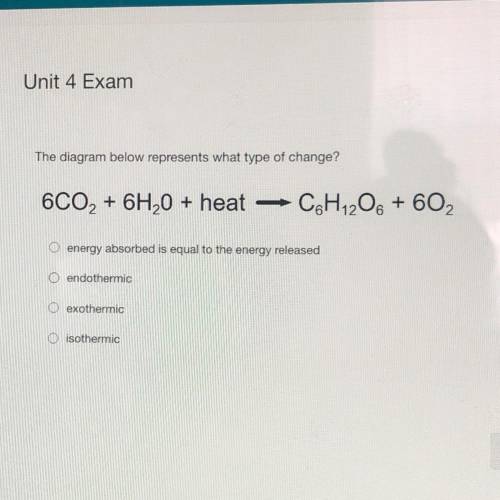
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
O energy absorbed is equal to the energy released
O endothermic
exothermic
O isothermic

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The ph of carrots are 5.0 how it is classified a.acidic b.basic c.indicator d.neutral
Answers: 2

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 17:10
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
You know the right answer?
The diagram below represents what type of change?
6CO2 + 6H2O + heat
C&H 206 + 602
...
C&H 206 + 602
...
Questions

Mathematics, 28.05.2020 01:03

Mathematics, 28.05.2020 01:03



Computers and Technology, 28.05.2020 01:03




Computers and Technology, 28.05.2020 01:03


Computers and Technology, 28.05.2020 01:03





Mathematics, 28.05.2020 01:03






