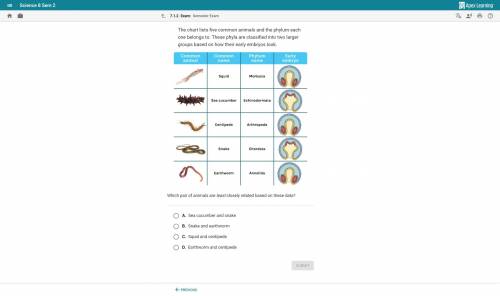
Help and will give PLEASE
...

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 18:10
Consider the following reaction at equilibrium: c(s)+h2o(g)⇌co(g)+h2(g) predict whether the reaction will shift left, shift right, or remain unchanged upon each of the following disturbances. a) c is added to the reaction mixture. b) h2ois condensed and removed from the reaction mixture c) co is added to the reaction mixture d) h2 is removed from the reaction mixture.
Answers: 3

Chemistry, 22.06.2019 21:30
In one or two grammatically correct sentences, write a definition for the term molecule geometry
Answers: 3

Chemistry, 22.06.2019 23:10
Amines are good nucleophiles, even though they are neutral molecules. how would the rate of an sn2 reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? amines are good nucleophiles, even though they are neutral molecules. how would the rate of an reaction between an amine and an alkyl halide be affected if the polarity of the solvent is increased? because both reactants in the rate-limiting step are neutral, the reaction will be faster if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will be slower if the polarity of the solvent is increased. because both reactants in the rate-limiting step are neutral, the reaction will occur at the same rate if the polarity of the solvent is increased. request answer
Answers: 3
You know the right answer?
Questions

Mathematics, 01.12.2020 14:50

Arts, 01.12.2020 14:50


English, 01.12.2020 14:50


Spanish, 01.12.2020 14:50


Health, 01.12.2020 14:50

English, 01.12.2020 14:50


English, 01.12.2020 15:00

Chemistry, 01.12.2020 15:00

English, 01.12.2020 15:00

Social Studies, 01.12.2020 15:00



World Languages, 01.12.2020 15:00


Physics, 01.12.2020 15:00

English, 01.12.2020 15:00