
Chemistry, 04.03.2021 07:10 sierram298
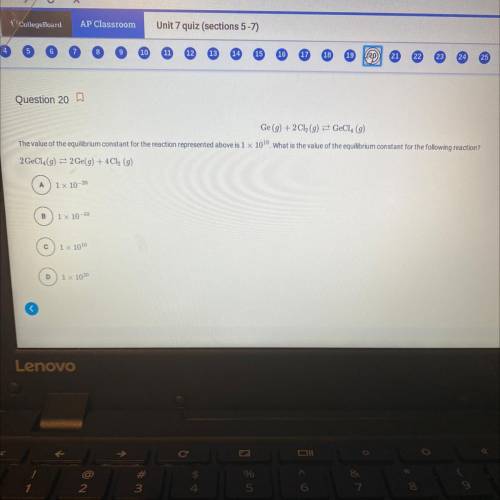
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented above is 1 x 100. What is the value of the equilibrium constant for the following reaction?
2 GeCl (g) = 2 Ge(g) +4Cl (g)
a) 1x 10-20
b) 1x 10-10
c) 1x 1010
d) 1 x 1020
will give brainliest !!

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of each individual carbon atom in c2o42−?
Answers: 1
You know the right answer?
Ge (g) + 2Cl(g) = GeCl4 (g)
The value of the equilibrium constant for the reaction represented abov...
Questions





Computers and Technology, 14.01.2020 04:31

History, 14.01.2020 04:31





English, 14.01.2020 04:31






Mathematics, 14.01.2020 04:31



Biology, 14.01.2020 04:31



