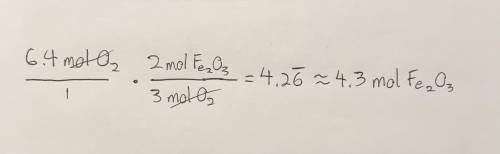Chemistry, 04.03.2021 01:10 lokaranjan5736
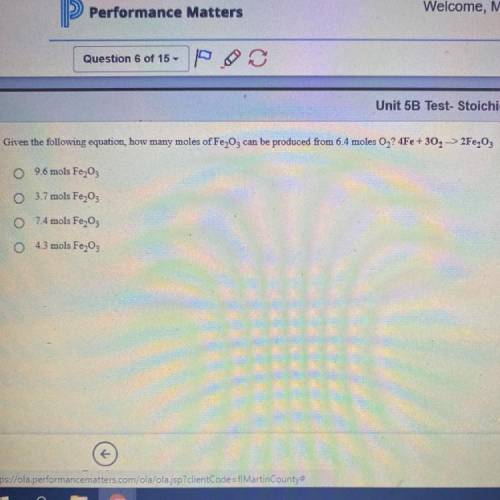
Given the following equation, how many moles of Fe2O3 can be produced from 6.4 moles O2? 4Fe +3O2 - - > 2Fe2O3

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:04
Brad and his family like to go camping. they always take a gas lantern with them. the lantern can take fiuels but always puts out the same amount of light. brad has two different kinds of fuel for the lantern. how can he figure out which kind of fuel has more chemical energy that the lantern can turn into light? measure the brightness of the lantern using each kind of fuel by comparing it to the full moon. g measure the volume of the containers of fuel. measure how long the lantern stays lit on a fixed amount of fuel. measure the mass of each container of fuel using a balance scale. answer 12
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1
You know the right answer?
Given the following equation, how many moles of Fe2O3 can be produced from 6.4 moles O2?
4Fe +3O2 -...
Questions

History, 22.11.2020 08:50

Computers and Technology, 22.11.2020 08:50


Mathematics, 22.11.2020 08:50

Mathematics, 22.11.2020 08:50

Mathematics, 22.11.2020 08:50

Mathematics, 22.11.2020 08:50

Biology, 22.11.2020 08:50



English, 22.11.2020 08:50



English, 22.11.2020 08:50




Physics, 22.11.2020 08:50

Mathematics, 22.11.2020 09:00

Mathematics, 22.11.2020 09:00