
Chemistry, 03.03.2021 23:50 Snowball080717
If excess sodium sulfate react with 36 g of iron (III) phosphate and produce a 65.0% yield of sodium phosphate, how many grams of sodium phosphate are actually produced?
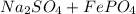 ->
-> 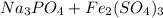

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1


Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2
You know the right answer?
If excess sodium sulfate react with 36 g of iron (III) phosphate and produce a 65.0% yield of sodium...
Questions

Mathematics, 04.12.2019 11:31


Physics, 04.12.2019 11:31




Computers and Technology, 04.12.2019 11:31



Mathematics, 04.12.2019 11:31


Mathematics, 04.12.2019 11:31

Mathematics, 04.12.2019 11:31


English, 04.12.2019 11:31

Advanced Placement (AP), 04.12.2019 11:31


Mathematics, 04.12.2019 11:31


Engineering, 04.12.2019 11:31



