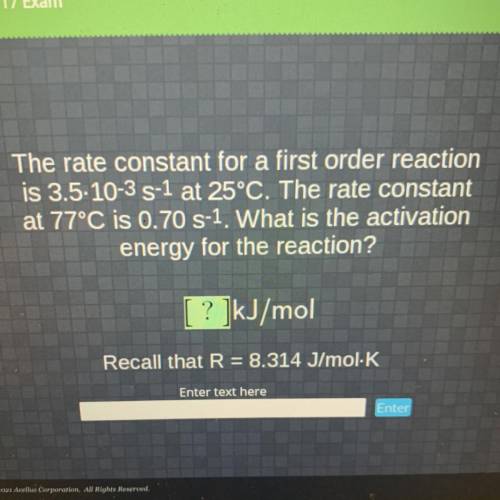
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77...

Chemistry, 03.03.2021 22:00 ryanzl1291
The rate constant for a first order reaction
is 3.5-10-3 S-1 at 25°C. The rate constant
at 77°C is 0.70 S-1. What is the activation
energy for the reaction?
[ ? ]kJ/mol
Recall that R = 8.314 J/mol K
-hurry it’s a test

Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 00:00
How many peaks will be present in a mass spectrum for brcl?
Answers: 1

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 20:00
Within nonmetals,ions that have more electrons tend to be bigger than ions that have fewer additional electrons why?
Answers: 1
You know the right answer?
Questions

Mathematics, 05.05.2020 20:28


History, 05.05.2020 20:28


Health, 05.05.2020 20:28



Mathematics, 05.05.2020 20:28


Mathematics, 05.05.2020 20:28




Mathematics, 05.05.2020 20:28



Mathematics, 05.05.2020 20:28


Mathematics, 05.05.2020 20:28



