
Chemistry, 03.03.2021 21:10 obliviousho2018
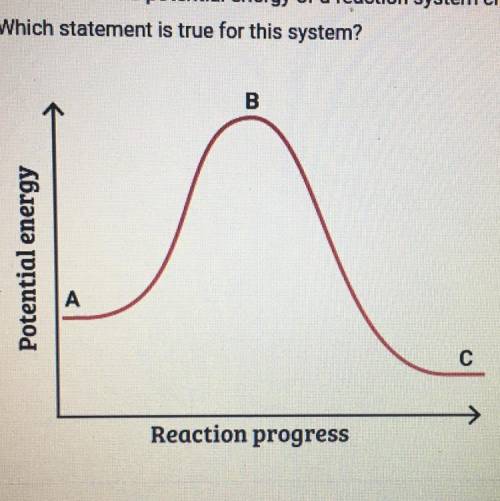
This graph shows how the potential energy of a reaction system changes
over time. Which statement is true for this system
A. The potential energy of the reactants is greater than the potential
energy of the products.
B. The height of the curve at point A represents the activation energy.
C. The height of the curve at point B represents the activation energy.
D. The potential energy of the products is greater than the potential
energy of the reactants.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1


Chemistry, 23.06.2019 14:30
Which statement best identifies the process shown? the process must be fusion because energy is released. a.the process must be fusion because a heavier nucleus forms from smaller nuclei. b.the process must be fission because a large nucleus breaks into smaller nuclei. c.the process must be fission because neutrons are formed.
Answers: 1
You know the right answer?
This graph shows how the potential energy of a reaction system changes
over time. Which statement i...
Questions








Mathematics, 26.03.2021 06:20



Chemistry, 26.03.2021 06:20

Biology, 26.03.2021 06:20

Mathematics, 26.03.2021 06:20

English, 26.03.2021 06:20



Mathematics, 26.03.2021 06:20

Business, 26.03.2021 06:20




