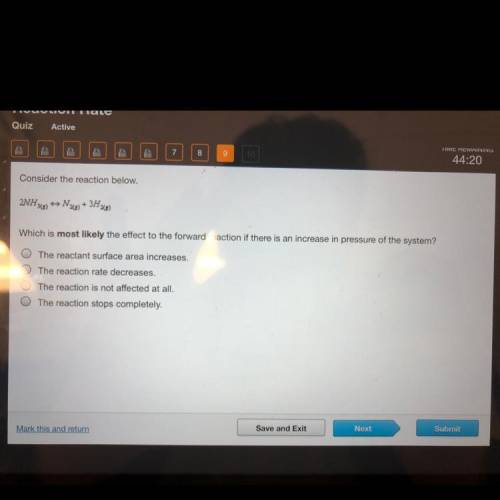
Consider the reaction below.
2NH308) N28) + 3H22)
Which is most likely the effect to the forw...

Chemistry, 03.03.2021 17:10 butterflycc
Consider the reaction below.
2NH308) N28) + 3H22)
Which is most likely the effect to the forward reaction if there is an increase in pressure of the system?
The reactant surface area increases.
The reaction rate decreases.
O The reaction is not affected at all.
The reaction stops completely

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:20
What is the formula for the compound dinitrogen pentoxide? a. n4o5 b. n5o4 c. n4o6 d. n5o2 e. n2o5
Answers: 3

Chemistry, 23.06.2019 00:30
What is the percent by mass of magnesium sulfate in mgso4.7h2o
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 09:00
Chortling is used to clean water. another possible atom that would also work is a. sodium b. sulfur c. bromine
Answers: 1
You know the right answer?
Questions

Social Studies, 23.01.2021 05:40

English, 23.01.2021 05:40

Mathematics, 23.01.2021 05:40

English, 23.01.2021 05:40

Law, 23.01.2021 05:40


Advanced Placement (AP), 23.01.2021 05:40



History, 23.01.2021 05:40

Mathematics, 23.01.2021 05:40



Business, 23.01.2021 05:40

Law, 23.01.2021 05:40


English, 23.01.2021 05:40

Chemistry, 23.01.2021 05:40

Biology, 23.01.2021 05:40

Mathematics, 23.01.2021 05:40




