
Chemistry, 03.03.2021 14:00 estebanmff
How many moles of water formed from 3.4 moles of oxygen?
2. How many moles of carbon dioxide produced from 5.2 moles of butane?
3. How many moles of water produced from 25.6 moles of butane?
4Na + O2 à 2Na2O Use this equation to answer questions 4 and 5.
4. How many moles of oxygen are required to completely react with 9.5 g of sodium?
5. How many grams of sodium are required to produce 12.5 grams of sodium oxide?
2NaOH + H2SO4 à 2H2O + Na2SO4
6. How many moles of sodium sulfate are formed if you start from 200 grams of sodium hydroxide and an excess of sulfuric acid?
7. If 8 g of sodium sulfate was produced from the reaction shown how many grams of sulfuric acid was used as a reactant?
Pb(SO4)2 + 4LiNO3 à Pb(NO3)4 + 2Li2SO4
8. How many grams of lithium nitrate will be needed to make 250 grams of lead (lV) nitrate assuming that you have adequate amount of lithium nitrate?
2Fe2O3 + 3C à 4Fe + 3CO2
9 Calculate the theoretical yield of iron in grams produced from 5.67 g of iron (lll) oxide.
10. Calculate the number of atoms of iron produced from 100.0 g of iron (lll) oxide.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2
You know the right answer?
How many moles of water formed from 3.4 moles of oxygen?
2. How many moles of carbon dioxide produc...
Questions

Mathematics, 31.01.2022 22:50

Mathematics, 31.01.2022 22:50


Mathematics, 31.01.2022 22:50


English, 31.01.2022 22:50

Mathematics, 31.01.2022 22:50

Mathematics, 31.01.2022 23:00

Mathematics, 31.01.2022 23:00



Mathematics, 31.01.2022 23:00

Mathematics, 31.01.2022 23:00

Biology, 31.01.2022 23:00

Mathematics, 31.01.2022 23:00


Mathematics, 31.01.2022 23:00



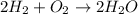
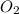 produces = 2 moles of
produces = 2 moles of 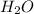
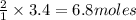 of
of 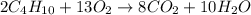
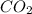
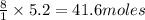 of
of 

