Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:20
The yearly amounts of carbon emissions from cars in belgium are normally distributed with a mean of 13.9 gigagrams per year and a standard deviation of 5.8 gigagrams per year. find the probability that the amount of carbon emissions from cars in belgium for a randomly selected year are between 11.5 gigagrams and 14.0 gigagrams per year. a. 0.340 b. 0.660 c. 0.167 d. 0.397
Answers: 2

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
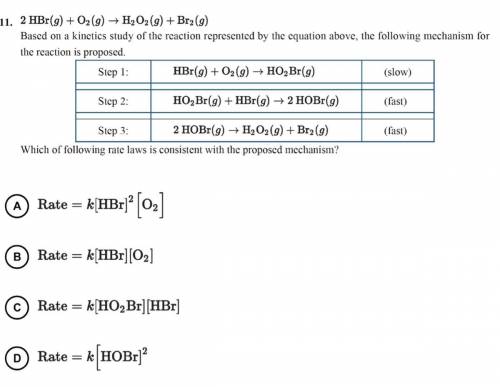
2 HBr(g)+O2(g)—>H2O2(g)+Br2(g)
Based on a kinetics study of the reaction represented by the equ...
Questions


Mathematics, 26.09.2019 16:30






Geography, 26.09.2019 16:30

Advanced Placement (AP), 26.09.2019 16:30



History, 26.09.2019 16:30





Mathematics, 26.09.2019 16:30