
Chemistry, 02.03.2021 22:00 carolinehodges
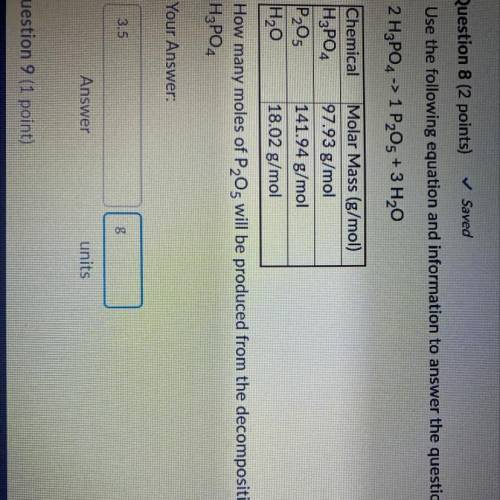
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205 + 3 H20
Chemical
Molar Mass (g/mol)
| H₂PO4 97.93 g/mol
P₂O5 141.94 g/mol
H2O 18.02 g/mol
How many moles of P2O5 will be produced from the decomposition of 0.833 mol of
H3PO4

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Use the following equation and information to answer the question that follows
2 H3PO4 -> 1 P205...
Questions

Mathematics, 08.06.2021 16:40


History, 08.06.2021 16:40

Business, 08.06.2021 16:40



Physics, 08.06.2021 16:40


Mathematics, 08.06.2021 16:40


World Languages, 08.06.2021 16:40

Mathematics, 08.06.2021 16:40


Social Studies, 08.06.2021 16:40



Mathematics, 08.06.2021 16:40


Mathematics, 08.06.2021 16:40

Mathematics, 08.06.2021 16:40



