
Chemistry, 02.03.2021 21:40 jefersonzoruajas
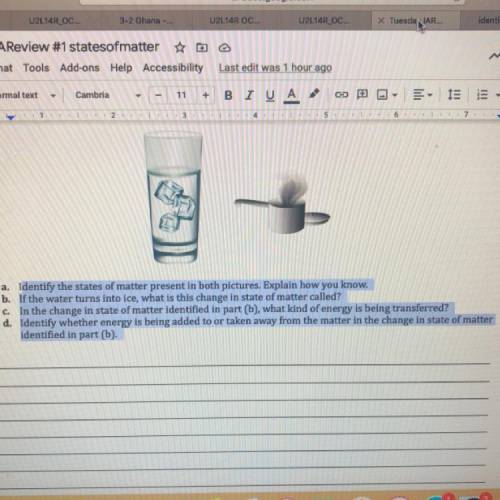
A. Identify the states of matter present in both pictures. Explain how you know.
b. If the water turns into ice, what is this change in state of matter called?
In the change in state of matter identified in part (b), what kind of energy is being transferred?
d. Identify whether energy is being added to or taken away from the matter in the change in state of matter
identified in part (b).
c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 21.06.2019 17:00
What is important to study for nios grade 12 chemistry? i have only one month left.
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1
You know the right answer?
A. Identify the states of matter present in both pictures. Explain how you know.
b. If the water tu...
Questions



Chemistry, 04.07.2019 05:30


Mathematics, 04.07.2019 05:30


Biology, 04.07.2019 05:30

Biology, 04.07.2019 05:30


Mathematics, 04.07.2019 05:30







Health, 04.07.2019 05:30

English, 04.07.2019 05:30




