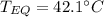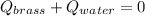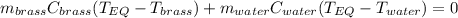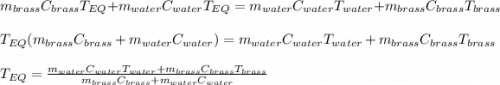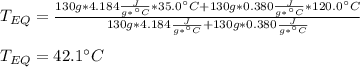Chemistry, 02.03.2021 14:00 LarryJoeseph
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g of water at 35.0 degrees Celsius. Disregard the absorption of heat by the cup and
calculate the final temperature of the brass and water. Specific heat of water = 4.18 J/gC,
specific heat of brass=0.380 J/gC. Attach your complete solution

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2

Chemistry, 23.06.2019 02:00
The bohr model of the atom explained why emission spectra are discrete. it could also be used to explain the photoelectric effect. which is a correct explanation of the photoelectric effect according to the model?
Answers: 3

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1
You know the right answer?
A 130 g sample of brass at 120.0 degrees Celsius is placed in a calorimeter cup that contains
130 g...
Questions



Mathematics, 29.09.2020 14:01

Social Studies, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01


History, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

History, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Geography, 29.09.2020 14:01



Mathematics, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01

Geography, 29.09.2020 14:01

Computers and Technology, 29.09.2020 14:01

Mathematics, 29.09.2020 14:01