
Chemistry, 02.03.2021 14:00 camiserjai1832
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
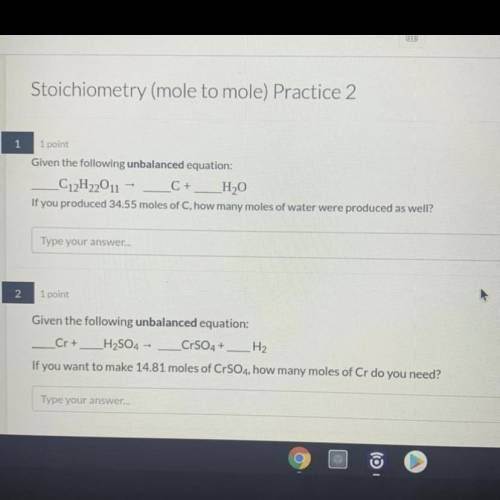
1. Given the unbalanced equation:
C12H22O11 = C + H2O
If you produced 34.55 moles of C, how many moles of water were produced as well?
2. Given the unbalanced equation:
Cr + H2SO4 = CrSO4 + H2
If you want to make 14.81 moles of CrSO4, how many moles of Cr do you need?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:50
It has been two weeks since charles met with daniel, a dietitian, who provided charles with a menu for weight loss. charles and his mother are going back to see daniel again with a chart of the food charles has eaten. the following lists what charles ate in one day: breakfast 1 banana, 1 cup of nonfat milk, 1 egg lunch 1 cup of carrots, 3 oz of steak, 1 apple, 1 cup of nonfat milk dinner 6 oz of skinless chicken, 1 baked potato, 3 oz of broccoli, 1 cup of nonfat milk
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1


Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
Ok we trying this again. so just in case if the picture isnt showing, here are the questions:
1. Gi...
Questions

Mathematics, 03.12.2019 07:31

Social Studies, 03.12.2019 07:31

History, 03.12.2019 07:31

English, 03.12.2019 07:31

Biology, 03.12.2019 07:31


History, 03.12.2019 07:31


English, 03.12.2019 07:31




Health, 03.12.2019 07:31

Physics, 03.12.2019 07:31

History, 03.12.2019 07:31


Biology, 03.12.2019 07:31



Mathematics, 03.12.2019 07:31



