
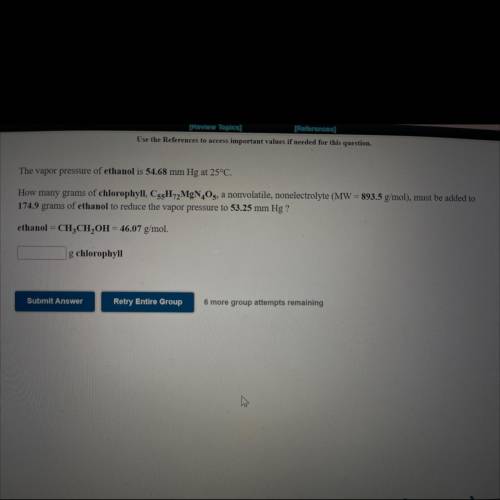
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5, a nonvolatile, nonelectrolyte (MW = 893.5 g/mol), must be added to
174.9 grams of ethanol to reduce the vapor pressure to 53.25 mm Hg ?
ethanol = CH3CH2OH = 46.07 g/mol.
How many ___g chlorophyll?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2


Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
The vapor pressure of ethanol is 54.68 mm Hg at 25°C.
How many grams of chlorophyll, C55H72MgN4O5,...
Questions

English, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01

Geography, 01.09.2020 07:01

Chemistry, 01.09.2020 07:01

Social Studies, 01.09.2020 07:01




Social Studies, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01

History, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01

History, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01


English, 01.09.2020 07:01


Mathematics, 01.09.2020 07:01

Mathematics, 01.09.2020 07:01



