
Chemistry, 02.03.2021 07:20 sharnisefrazier
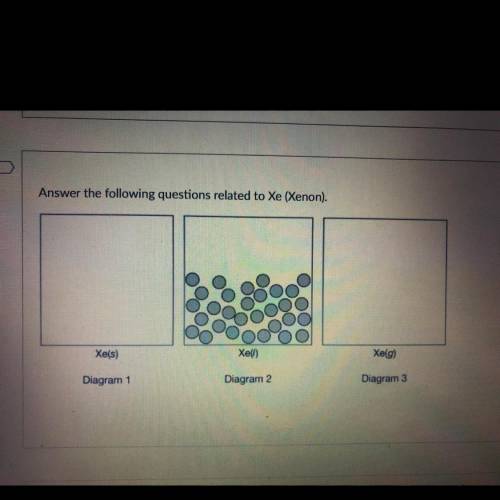
Let P and V represent the pressure and volume of the Xe (g) in the container in diagram 3. If a piston is used to reduce the volume of the gas to V 2 at a constant temperature, what is the new pressure in the container in terms of the original pressure, P?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2
You know the right answer?
Let P and V represent the pressure and volume of the Xe (g) in the container in diagram 3. If a pist...
Questions


History, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30


Biology, 29.03.2021 22:30


Mathematics, 29.03.2021 22:30




Mathematics, 29.03.2021 22:30


Mathematics, 29.03.2021 22:30


SAT, 29.03.2021 22:30

Mathematics, 29.03.2021 22:30



