Chemistry, 01.03.2021 23:20 junior2461
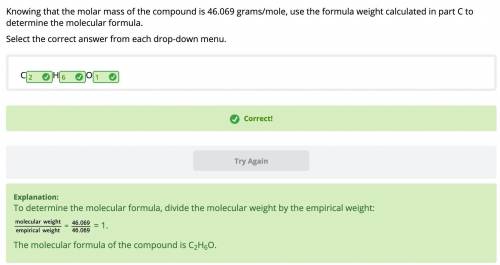
Knowing that the molar mass of the compound is 46.069 grams/mole, use the formula weight calculated in part C to
determine the molecular formula.
Select the correct answer from each drop-down menu.
С_H_O_

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 23.06.2019 00:00
2-bromo-2-methylbutane undergoes an e1 elimination reaction in the presence of ethanol. in the next reaction only one of the possible products is represented. although the product shown is not the major product of the reaction, notice that there is more than one way it can be produced. complete the mechanism and draw the missing substances.
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1
You know the right answer?
Knowing that the molar mass of the compound is 46.069 grams/mole, use the formula weight calculated...
Questions




Arts, 04.05.2020 22:37



Chemistry, 04.05.2020 22:37


Mathematics, 04.05.2020 22:37


Mathematics, 04.05.2020 22:37

Mathematics, 04.05.2020 22:37



Mathematics, 04.05.2020 22:37


Mathematics, 04.05.2020 22:37