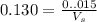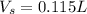Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1
You know the right answer?
What volume of 0.130 M HCl is required for the complete neutralization of 1.30 g of NaHCO3 (sodium b...
Questions

Mathematics, 22.10.2019 16:00

History, 22.10.2019 16:00


Mathematics, 22.10.2019 16:00


Social Studies, 22.10.2019 16:00




Chemistry, 22.10.2019 16:00


Biology, 22.10.2019 16:00

History, 22.10.2019 16:00

History, 22.10.2019 16:00




Mathematics, 22.10.2019 16:00

Mathematics, 22.10.2019 16:00

History, 22.10.2019 16:00

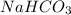 =
= 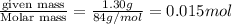

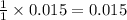 mole of HCl
mole of HCl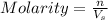
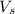 = volume of solution in L
= volume of solution in L