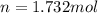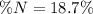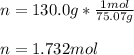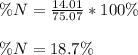Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:00
One significant difference between an ionic bond, where electrons are taken from one atom and added to another atom, and a covalent or metallic bond, where electrons are shared is
Answers: 2


Chemistry, 22.06.2019 20:00
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1

Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2
You know the right answer?
1. Calculate how many moles of glycine are in a 130.0-g sample of glycine.2. Calculate the percent n...
Questions

Social Studies, 19.04.2021 18:10


Mathematics, 19.04.2021 18:10





Social Studies, 19.04.2021 18:10






Chemistry, 19.04.2021 18:10




Biology, 19.04.2021 18:10


History, 19.04.2021 18:10