
Chemistry, 01.03.2021 21:50 lanaiheart7
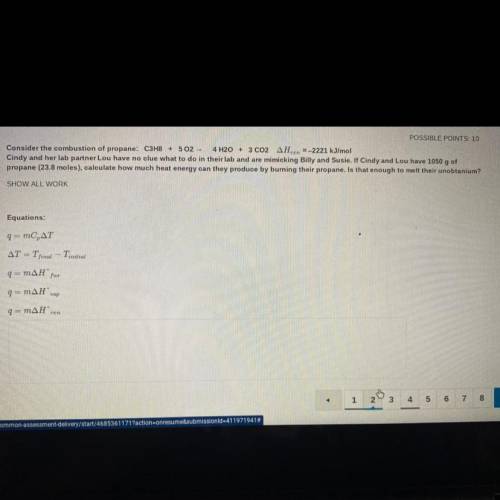
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab partner Lou have no clue what to do in their lab and are mimicking Billy and Susie. If Cindy and Lou have 1050 g of
propane (23.8 moles), calculate how much heat energy can they produce by burning their propane. Is that enough to melt their unobtanium?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1
You know the right answer?
Consider the combustion of propane: C3H8 + 502 - 4 H2O + 3 CO2 AHzn =-2221 kJ/mol
Cindy and her lab...
Questions

History, 23.11.2019 23:31

Social Studies, 23.11.2019 23:31

Geography, 23.11.2019 23:31

Mathematics, 23.11.2019 23:31



Social Studies, 23.11.2019 23:31


Spanish, 23.11.2019 23:31

Mathematics, 23.11.2019 23:31

Biology, 23.11.2019 23:31



English, 23.11.2019 23:31


Mathematics, 23.11.2019 23:31






