PLEASE HELP ASAP I WILL GIVE BRAINLIEST!
Consider the reaction pathway graph below.
The rate...

Chemistry, 01.03.2021 19:00 fixianstewart
PLEASE HELP ASAP I WILL GIVE BRAINLIEST!
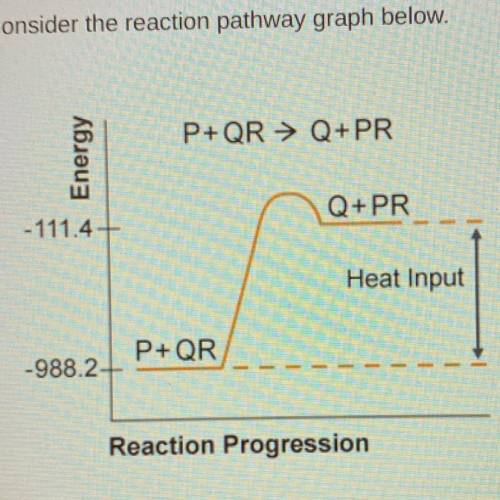
Consider the reaction pathway graph below.
The rate increases by a factor of 9 when the concentration of A triples. The rate triples when the concentration of B triples. What is the new rate law for the reaction?
A) endothermic because Hrxn=-876.8 kJ
B) endothermic because Hrxn=876.8kJ
C)exothermic because Hrxn= -1099.6kJ
D) exothermic because Hrxn=1099.6kJ

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1


Chemistry, 23.06.2019 18:30
Describe two techniques used to measure the ph of a solution
Answers: 2

Chemistry, 24.06.2019 00:30
When a pendulum is held high and taut and then is released, the pendulum begins to swing what's the correct order of the energytransformations in this example? (pe stands for potential energya.gravitational peelastic pethermal energyb.gravitational pe - kinetic energythermal energyc.kinetic energyelastic pe - thermal energyd.kinetic energy-gravitational pethermal energye. thermal energy - gravitational pekinetic energy
Answers: 1
You know the right answer?
Questions


Mathematics, 13.05.2021 05:00

Spanish, 13.05.2021 05:00



Mathematics, 13.05.2021 05:00







Mathematics, 13.05.2021 05:00

Social Studies, 13.05.2021 05:00

Mathematics, 13.05.2021 05:00


Mathematics, 13.05.2021 05:00


Physics, 13.05.2021 05:00

English, 13.05.2021 05:00



