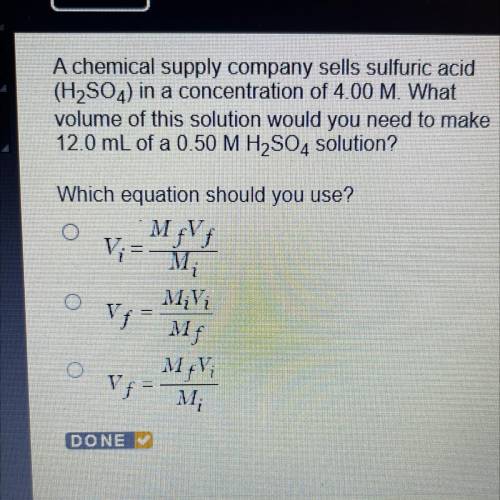
A chemical supply company sells sulfuric acid
(H2SO4) in a concentration of 4.00 M. What
volu...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Why does earth rotate? because earth is formed from cold gases collapsing due to gravity because the matter in the nebula that formed earth was spinning because earth forms more than 99% of the mass of the solar system because the hydrogen atoms inside the nebula fused to form helium
Answers: 1

Chemistry, 23.06.2019 00:30
What is the chemical formula of magnesium bromide? a. mgbr2 b. mgbr c. mg2br2 d. mg2br
Answers: 3

Chemistry, 23.06.2019 10:20
Determine the mass of the object below with accuracy and to the correct degree of precision. a. 324.2 g b. 324 g c. 324.30 g d. 324.25 g
Answers: 3

Chemistry, 23.06.2019 11:30
Which of these have the same number of particles as 1 mole of water h2o
Answers: 1
You know the right answer?
Questions






Mathematics, 07.09.2020 02:01

Computers and Technology, 07.09.2020 02:01




Computers and Technology, 07.09.2020 02:01

Advanced Placement (AP), 07.09.2020 02:01

English, 07.09.2020 02:01


Mathematics, 07.09.2020 02:01



Mathematics, 07.09.2020 02:01

Computers and Technology, 07.09.2020 02:01

Computers and Technology, 07.09.2020 02:01