Type of Solutions and Factors that Affect
them
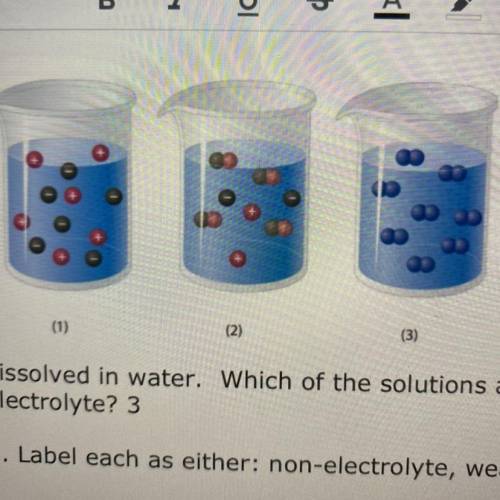
1. The diagram above represents solute partic...

Type of Solutions and Factors that Affect
them
1. The diagram above represents solute particles
dissolved in water. Which of the solutions above
(1,2,3) would be considered an electrolyte?
2. Label each as either: non-electrolyte, weak
electrolyte, or strong electrolyte
(1)
(2)
(3)
2. A chemist dissolves solid potassium chloride in a given amount of water until no more will
dissolve at that temperature. How could that solution be described?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 22.06.2019 23:30
How many grams of ammonia would be produced by the decomposition of 16.93 mlof hydrazine? (the density of hydrazine is 1.021g/ml)
Answers: 3

Chemistry, 23.06.2019 12:00
Which element has the largest atomic radius? a. asb. nc. pd. sb
Answers: 2
You know the right answer?
Questions


Spanish, 30.01.2020 18:47


World Languages, 30.01.2020 18:47



Mathematics, 30.01.2020 18:47

Mathematics, 30.01.2020 18:47

Computers and Technology, 30.01.2020 18:47

Mathematics, 30.01.2020 18:47


Mathematics, 30.01.2020 18:47

History, 30.01.2020 18:47




Geography, 30.01.2020 18:47






