
Chemistry, 26.02.2021 20:50 alexmoy45p8yd7v
PLEASE HELP EZ POINTS The reaction below releases enormous amounts of energy that could be used to provide power to homes and businesses.
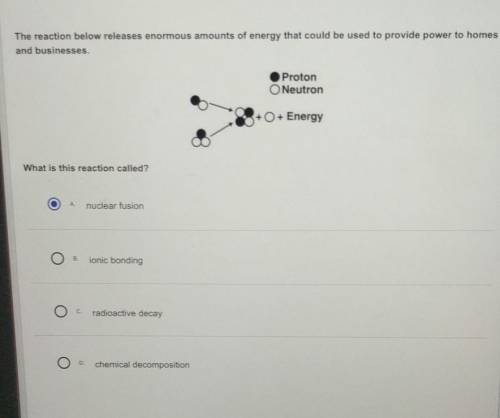

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 09:00
Which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 3

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

You know the right answer?
PLEASE HELP EZ POINTS
The reaction below releases enormous amounts of energy that could be used to...
Questions

Mathematics, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20


Social Studies, 17.12.2020 18:20


Mathematics, 17.12.2020 18:20




Arts, 17.12.2020 18:20


Chemistry, 17.12.2020 18:20


Mathematics, 17.12.2020 18:20


Mathematics, 17.12.2020 18:20

Spanish, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20

Mathematics, 17.12.2020 18:20



