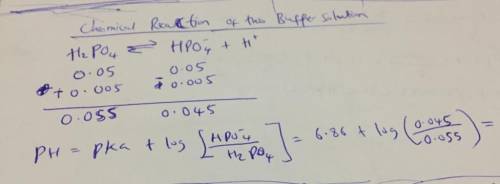Chemistry, 26.02.2021 06:00 jadabecute3739
what would the ph be at the end of an enzyme-catalyzed reaction if it were carried out in a 0.1 m phosphate buffer of pka 6.86 and 0.005 m of acid was produced during the reaction

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
what would the ph be at the end of an enzyme-catalyzed reaction if it were carried out in a 0.1 m ph...
Questions

Mathematics, 27.03.2020 01:04

Computers and Technology, 27.03.2020 01:04

English, 27.03.2020 01:04

History, 27.03.2020 01:04

Social Studies, 27.03.2020 01:04

Biology, 27.03.2020 01:04


Mathematics, 27.03.2020 01:04




Mathematics, 27.03.2020 01:04

Mathematics, 27.03.2020 01:04


History, 27.03.2020 01:04


Chemistry, 27.03.2020 01:04

Mathematics, 27.03.2020 01:04

Biology, 27.03.2020 01:04

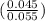 =
=