
Chemistry, 26.02.2021 04:40 gabrielleteti
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction?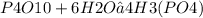

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 23.06.2019 04:31
2ki + pb(no3)2 → 2kno3 + pbi2 determine how many moles of kno3 are created if 0.03 moles of ki are completely consumed.
Answers: 1


Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
How many moles of H3(PO4) are produced when 71.0 g P4O10 reacts
completely with H2O in the reaction...
Questions

Mathematics, 12.02.2021 18:00

Medicine, 12.02.2021 18:00

English, 12.02.2021 18:00

Mathematics, 12.02.2021 18:00



Mathematics, 12.02.2021 18:00


Mathematics, 12.02.2021 18:00

Social Studies, 12.02.2021 18:00


Mathematics, 12.02.2021 18:00

Physics, 12.02.2021 18:00





Social Studies, 12.02.2021 18:00


World Languages, 12.02.2021 18:00



