
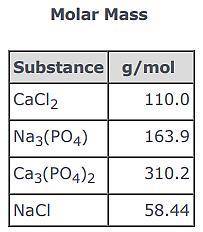
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass table above to answer the following question.
Suppose 163.9 g of Na3(PO4) reacted with sufficient CaCl2 in solution to actually yield 116 g of Ca3(PO4)2(s) . What is the percent yield of Ca3(PO4)2(s)?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which part of a feedback mechanism is able to monitor the conditions outside of cells and usually uses nerve cells to relay this information to an intergrating center
Answers: 2

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2
You know the right answer?
3CaCl2(aq) + 2Na3(PO4)(aq) → Ca3(PO4)2(s) + 6NaCl(aq)
Use the balanced equation and the Molar Mass...
Questions



History, 02.08.2021 07:10

Chemistry, 02.08.2021 07:10


Mathematics, 02.08.2021 07:10

Mathematics, 02.08.2021 07:10

English, 02.08.2021 07:10


Mathematics, 02.08.2021 07:10

English, 02.08.2021 07:10


Computers and Technology, 02.08.2021 07:10

English, 02.08.2021 07:10

Computers and Technology, 02.08.2021 07:10


Social Studies, 02.08.2021 07:10

Mathematics, 02.08.2021 07:10





