
Chemistry, 26.02.2021 02:00 hectorgonzalejr333
Question 1 (1 point)
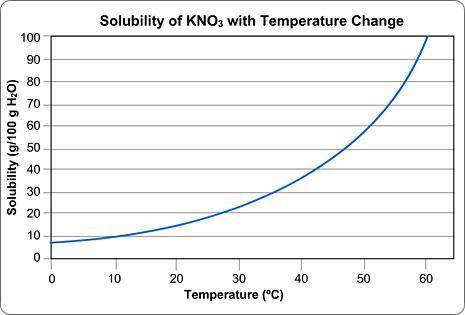
Above is a solubility curve for KNO3.
Solubility has nothing to do with the speed of dissolving; it's a measure of how much salt will dissolve at a given temperature.
The y-axis of the graph shows you how much KNO3 will dissolve in 100 g of water. In other words, it tells you the maximum amount of solute that will dissolve at different temperatures.
The x-axis tells you the minimum temperature needed to dissolve different amounts of KNO3 in 100 g of water.
Approximately how many grams of KNO3 will dissolve in 100 g water at 0 degrees Celsius?
Type in the number only; no units. Round your answer to the nearest whole number.
Question 1 options:
Question 2 (1 point)
Approximately what is the minimum temperature needed to dissolve 10 g of KNO3 in 100 g H2O?
Round your answer to the nearest whole number and type in the number only; no units.
Question 2 options:
Question 3 (1 point)
Imagine that you have 100g of water.
You start dissolving KNO3 in the water and you find that after you've dissolved about 55 g of KNO3, you can't dissolve any more; it just sinks to the bottom.
Approximately what is the temperature of the water?
Round your answer to the nearest whole number and submit the number only; no units.
Question 3 options:
Question 4 (1 point)
Saved
Increasing the temperature of the water will increase the amount of salt that it can dissolve.
Question 4 options:
True
False
Question 5 (1 point)
Saved
A solubility curve identifies how fast a solute will dissolve at different temperatures.
Question 5 options:
True
False
Question 6 (1 point)
Saved
This solubility curve indicates that at higher temperatures KNO3 will dissolve faster.
Question 6 options:
True
False
Question 7 (1 point)
Saved
A solubility curve tells you the minimum temperature needed to dissolve an amount of solute.
Question 7 options:
True
False

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 3

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

You know the right answer?
Question 1 (1 point)
Above is a solubility curve for KNO3.
Solubility has nothing to do...
Solubility has nothing to do...
Questions


Computers and Technology, 10.03.2020 03:05


Chemistry, 10.03.2020 03:05






Mathematics, 10.03.2020 03:05






Mathematics, 10.03.2020 03:05

Mathematics, 10.03.2020 03:05

Biology, 10.03.2020 03:05




