Chemistry, 26.02.2021 01:20 hugbug2554
We place 3.2 mol PCl5 in a 2.0 L flask and allow it to reach equilibrium at a given temperature. What is the final concentration of Cl2 in the flask?
PCl5(g) PCl3(aq) + Cl2(g)
Kc = 0.47
A) 0.55 M
B) 0.27 M
C) 0.66 M
D) 0.11 M
E) 0.32 M

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
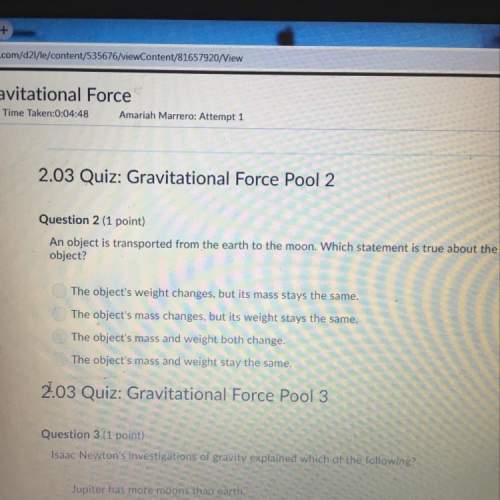
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3


Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3
You know the right answer?
We place 3.2 mol PCl5 in a 2.0 L flask and allow it to reach equilibrium at a given temperature. Wha...
Questions

Business, 20.07.2019 04:50

Mathematics, 20.07.2019 04:50

Mathematics, 20.07.2019 04:50


Biology, 20.07.2019 04:50

Social Studies, 20.07.2019 04:50

History, 20.07.2019 04:50

Social Studies, 20.07.2019 04:50





Mathematics, 20.07.2019 04:50

Business, 20.07.2019 04:50

Health, 20.07.2019 04:50



History, 20.07.2019 04:50

History, 20.07.2019 04:50