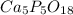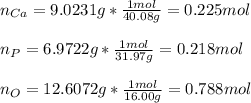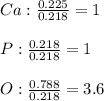Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identify the balanced chemical equation that represents a decomposition reaction. p4 + 3o2 ⟶ p2o3 2fe(oh)3 ⟶ 2feo3 + h2o cuso4 ⟶ cuo + 2so3 2fe(oh)3 ⟶ fe2o3 + 3h2o
Answers: 1



Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3
You know the right answer?
A sample of a compound used to polish dentures and as a nutrient and dietary supplement is analyzed...
Questions

Health, 21.03.2020 12:05

Mathematics, 21.03.2020 12:05

Mathematics, 21.03.2020 12:05


Mathematics, 21.03.2020 12:05



Mathematics, 21.03.2020 12:05

Biology, 21.03.2020 12:05

Mathematics, 21.03.2020 12:05

Chemistry, 21.03.2020 12:06

History, 21.03.2020 12:06

English, 21.03.2020 12:06


English, 21.03.2020 12:06


Social Studies, 21.03.2020 12:06


History, 21.03.2020 12:06